Details of the Drug
General Information of Drug (ID: DM5NM6E)
| Drug Name |
NADH
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
DPNH; Dihydrodiphosphopyridine nucleotide; Dihydronicotinamide adenine dinucleotide; Diphosphopyridine nucleotide reduced; NADH dianion; Nicotinamide adenine dinucleotide reduced; Reduced Nicotinamide Adenine Dinucleotide; NADH2; Beta-DPNH; Beta-NADH; Coenzyme I, reduced; Cozymase I, reduced; Diphosphopyridine nucleotide,reduced form; NAD-reduced; Nicotinamide adenine dinucleotide, reduced form; Reduced nicotinamide-adenine dinucleotide; Beta-Nicotinamide adenine dinucleotide, reduced dipotassium salt; NADH+H+; Nicotinaminde-Adenine-Dinucleotide; Adenosine pyrophosphate, 5'->5'-ester with 1,4-dihydro-1-beta-D-ribofuranosylnicotinamide (7CI); Adenosine 5'-(trihydrogen pyrophosphate), 5'->5'-ester with 1,4-dihydro-1-beta-D-ribofuranosylnicotinamide (8CI); Adenosine 5'-(trihydrogen diphosphate), P'->5'-ester with 1,4-dihydro-1-beta-D-ribofuranosyl-3-pyridinecarboxamide (9CI); Adenosine 5'-{3-[1-(3-carbamoyl-1,4-dihydropyridin-1-yl)-1,4-anhydro-D-ribitol-5-yl] dihydrogen diphosphate}; Adenosine 5'-{3-[1-(3-carbamoyl-1,4-dihydropyridin-1-yl)-1,4-anhydro-D-ribitol-5-yl] diphosphate}; [5-(6-Aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl [[5-(3-carbamoyl-4H-pyridin-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] hydrogen phosphate; [[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] [(2R,3S,4R,5R)-5-(3-carbamoyl-4H-pyridin-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl hydrogen phosphate; [(2R,3S,4R,5R)-5-(6-Aminopurin-9-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-[[(2R,3S,4R,5R)-5-(3-carbamoyl-4H-pyridin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-hydroxy-phosphoryl]oxy-phosphinic acid
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Dietary supplement
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
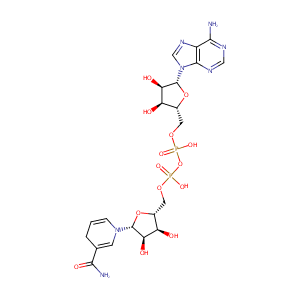 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 5 | Molecular Weight (mw) | 665.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -5.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 11 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 8 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 19 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
